Post by: OJM on October 22, 2014, 08:29:32 PM
I am currently synthesizing Tame (1,1,1-tris(amino-methyl)ethane) for my undergraduate research project.
I would greatly appreciate any advice from Inorganic & Organometallic chemist, or any who have experience working with azides. So Thank you in advance.
1,1,1-Tris(azido-methyl)ethane [10]:
A mixture of crude 1,1,1-(Benzenesulfonylchloride-methyl)ethane (50g) & NaN3 in Diethyleneglycol (170mL) is stirred and maintained under N2 @ 135° C for 16 hours. after cooling it is poured into water (340mL)
The resulting oil is collected and combined with diethyl ether (65mL) extract of the aqueous layer, and extracted with water.
The ethereal solution is is dried (Na2SO4), treated with charcoal, and evaporated.
Im trying to setup the apparatus in under a hood using N2 gas lines.
Thank you again for your time.
Post by: Hunter2 on October 23, 2014, 01:02:30 AM
I would assume Azid react with the -SO2-Cl group.
Post by: OJM on October 23, 2014, 01:35:25 AM
Post by: discodermolide on October 23, 2014, 01:55:40 AM
As you are making a tri-azido compound at 135°C what do you think may well happen here?
I don't see this as an undergraduate synthetic research project. So why do you need this compound?
Post by: OJM on October 23, 2014, 02:45:06 AM
^ 30.0 grams NaN3
After completing the azide substitution, the product in dry THF will be added slowly to a suspension of LiAlH4 (2Hr), then heated under reflux (18hr).-----> ***reduction of Polyazide to Polyamine
I specifically ask, How do i safely extract the resulting oil all while under the Nitrogen atmosphere.
I am a student in buffalo that has been working on this all semester.
Cheers
Post by: discodermolide on October 23, 2014, 03:22:19 AM
I ask because heating a tri-azide at 135°C may be a little unsafe.
If you have been working on this all summer then you should know how to do the extractions involved. And you should know the correct structures and their names as well.
Post by: Hunter2 on October 23, 2014, 03:30:38 AM
But how do you will get 1,1,1-Tris(azido-methyl)ethane .
1,1,1-(Benzenesulfonylchloride-methyl)ethane is for me also not clear how this should look like.
Post by: Dan on October 23, 2014, 05:14:00 PM
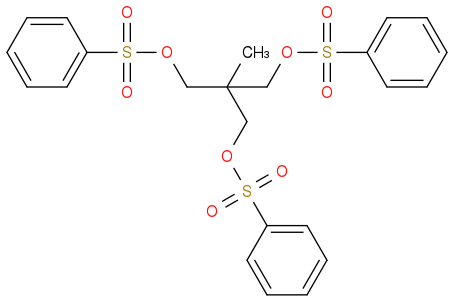
1,1,1-(Benzenesulfonylchloride-methyl)ethane
Be careful with your nomenclature - this does not exist. I assume you mean 1,1,1-tris(benzenesulfonyloxymethyl)ethane (which does not contain any chlorine)?
This one:
Be very very careful heating this to 170 °C. Make sure that is absolutely necessary as it could explode quite spectacularly on that scale. You (or a colleague) could be maimed or killed. I'm no azidophobe, I have worked with them quite extensively over the last 8 years, but I would never attempt that procedure at that temperature on that scale. I am 99.9% certain there is no way the safety office here would allow anyone to set it up anyway. My advice would be to speak to the safety office at your institution before doing it.
Post by: clarkstill on October 24, 2014, 02:30:04 AM
Be very very careful heating this to 170 °C. Make sure that is absolutely necessary as it could explode quite spectacularly on that scale. You (or a colleague) could be maimed or killed. I'm no azidophobe, I have worked with them quite extensively over the last 8 years, but I would never attempt that procedure at that temperature on that scale. I am 99.9% certain there is no way the safety office here would allow anyone to set it up anyway. My advice would be to speak to the safety office at your institution before doing it.
I couldn't agree more; you absolutely should not do this reaction. If you're desperate to make the triamine you could try a triple Gabriel synthesis? Or benzylamine and then reduction of the benzyl group? Or even just excess ammonia? Anyway you definitely should be not heating a molecule with 4 carbons and 9 nitrogens to 170C, neither should you be evaporating the product to dryness. If you're dead set on doing this reaction please let me know where in the world you are so I can arrange to be elsewhere.
Post by: mjc123 on October 24, 2014, 06:01:01 AM
Post by: OJM on October 26, 2014, 02:33:23 PM
Dan is right it should be,
" I assume you mean 1,1,1-tris(benzenesulfonyloxymethyl)ethane (which does not contain any chlorine)?"
We haven't started the reaction yet but i could upload a picture of the apparatus.
TAME has already been synthesized 3 times at my school a decade ago.The temperature never exceeds 135° C.
Post by: OJM on October 26, 2014, 02:39:08 PM
Post by: Dan on October 26, 2014, 05:44:45 PM
Some modifications to the apparatus need to be made. a soxhlet thimble apparatus and dean stark apparatus will be added.
I'd definitely add a blast shield as well if I had to do that experiment.
TAME has already been synthesized 3 times at my school a decade ago.The temperature never exceeds 135° C.
Just because it was done before a decade ago doesn't mean it's safe to do or is compatible with your school's current safety policies (which have probably tightened in the last 10 years).
You should consult with your safety officer(s) before doing the experiment to ascertain whether you can actually do it and, if you can, to make sure all reasonable precautions to minimize the risks are in place. This is probably a legal requirement. You could be in serious trouble (even if nobody is injured or if nothing goes wrong) if you are not operating according to health and safety law.
Post by: OJM on October 27, 2014, 01:16:18 PM
My professor has already consulted with the school safety office. I will be attempting a dry run to see if everything works first. I do have a blast shield but did not want to block the apparatus in the picture.
Ill post an update of the most current apparatus later today.
Post by: Enthalpy on November 02, 2014, 10:49:12 AM
- What prevents 30 batches with each 1g azide, instead of one batch with 30g?
- Could neopentane triol or trihalide react with ammonia to provide the triamine?
I just read (without understanding the implications) in Huntsmnn's brochure about ethyleneamines:
"Huntsman manufactures ethyleneamines by the ethylene dichloride/ammonia process. This process consists of the reaction of ethylene dichloride with ammonia, followed by neutralization with sodium hydroxide to produce a mixture of ethyleneamines and sodium chloride. The salt is removed from the amine mixture, and the individual amines are separated by fractional distillation."
They don't tell about the pressure, though.
Ill post an update of the most current apparatus later today.
OJM, is everything fine?
Post by: kriggy on November 02, 2014, 11:05:53 AM
- Could neopentane triol or trihalide react with ammonia to provide the triamine?
I would say yes but I suppose you get crazy mixture of products since after the amonia is alkylated first time, it is then more reactive towards another alkylation.
What about using gabriel reaction instead of direct alkylation?
Post by: Enthalpy on November 02, 2014, 01:00:29 PM
What about a large excess of NH3? Since all OH or X must be substituted, there is no hard limit to the proportions. For ethyleneamines, producers do get ethylenediamine as well, in useable proportion influenced by the reactants.The wild mixture of products must of course be separated.
Variant of the azide way: the available full text of
"Ligand Synthesis and Metal Complex Formation of 1,2,3-Triaminopropane"
states on its second page:
"Due to the potential hazards of polyazides, a procedure was developed that did not require the isolation of these intermediates as pure substances. The solutions of the azides were rather transferred directly into the autoclave and hydrogenated to give the polyamines".
Any applicable to TAME instead of TRAP?
Post by: kriggy on November 03, 2014, 06:14:22 AM
Post by: OJM on November 04, 2014, 12:17:27 AM
Post by: Enthalpy on November 08, 2014, 05:17:57 PM
The tribromo is far less common, no price given online.
How do they get the halo atoms at different carbons? Free radical halogenation would preferentially group them, or?