Post by: corax23 on November 21, 2014, 09:18:41 PM
I need help for this synthesis:
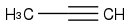 :rarrow:
:rarrow: 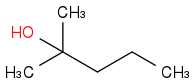
Thanks!
Post by: AromaticAcrobatic on November 21, 2014, 09:41:55 PM
:spinpaired:
Post by: orgopete on November 22, 2014, 11:24:04 AM
Post by: corax23 on November 22, 2014, 12:38:01 PM
Post by: kriggy on November 22, 2014, 12:39:52 PM
Post by: Altered State on November 22, 2014, 01:00:11 PM
I did it!! I can create a ketone with the reagent and H2O and I added the ketone to the acetylide.
Just some comments on that:
You can perfectly generate acetone from that alkyne, but actually, you wouldn't have to "create" a ketone, the one you need is just acetone, CH3C(=O)CH3, using propyne to make acetone would be a waste of time and money. But if you are asked to use that reagent as your only carbon source, is fine.
Also, then, mechanistically speaking, you add the acetylide to the ketone, not the other way around.