Post by: Shadow on December 10, 2014, 11:42:49 AM
Post by: OrgXemProf on December 10, 2014, 12:46:16 PM
You may be the first person today to express this desire, but surely you are aware that others have devoted considerable thought to this exercise in the past.
For example, see: "A Brief Discussion on Fenestranes", which can be found in J. A. Woolford, "The Double [3 + 2] Cycloaddition reaction", Springer-Verlag:Berlin, Chapter 3, pp 41-47.
Some questions/thoughts:
1. What is the likely hybridization of the central carbon atom in [4.4.4.4]fenestrane?
2. Can you cite any other examples of a known compound with a carbon atom that possesses similar hybridization and/or geometry?
3. You might have a look at "Structure and Energetics of [4.4.4.4]fenestrane": Shulman, J. M., et al., J. Am. Chem. Soc., 1983, 105, 743–744.
4. Rest secure in the knowledge that success in your proposed venture will lead to fame and fortune.
5. I would suggest that it may be too soon for you to purchase your new Maserati with the proceeds.
Post by: Shadow on December 10, 2014, 12:56:58 PM
Post by: OrgXemProf on December 10, 2014, 01:01:06 PM
Post by: OrgXemProf on December 10, 2014, 01:11:07 PM
You may be able to find this publication in a university chemistry (or science) library. Perhaps a glance at some of the individual chapters will lead to some fresh ideas on potential new target molecules for "weird synthesis".
Post by: curiouscat on December 10, 2014, 01:15:32 PM
Or are our theoretical methods not good enough yet & the only way to tell is to make one & see?
Post by: Shadow on December 11, 2014, 10:37:32 AM
Post by: discodermolide on December 11, 2014, 12:11:04 PM
In the case of "weird" molecules like this one is it possible to say whether it'd be a stable molecule even if one were to synthesize it?
Or are our theoretical methods not good enough yet & the only way to tell is to make one & see?
I think the theoretical methods are reasonably robust and some of these weird molecules were actually predicted to exist before they were actually synthesised. I may have a few examples, if I can find them.
Our colleague Enthalpy would know.
Post by: clarkstill on December 11, 2014, 12:47:54 PM
http://pubs.acs.org/doi/abs/10.1021/ja903914r
Post by: Shadow on December 11, 2014, 02:26:51 PM
Post by: Shadow on December 12, 2014, 11:59:17 AM
Post by: Enthalpy on December 14, 2014, 06:32:26 PM
For instance diasterane and triasterane look interesting but haven't been mass-produced up to now. Stellane may be nice. Is spiropentane, or better its dimer, available in railcar amounts?
Bicyclo[1.1.1]pentane, or better its (preferably unsymmetric) dimer or trimer (and their mixes), is definitely interesting. The precursor [1.1.1]propellane is obtained by photochemical methods, so the oligomer might just be a matter of mixing the propellane with the proper amount of illuminated iodine or bromine, then replacing the halocarbon with hydrogen.
By the way, last time I evaluated the lamp and electricity cost, photochemical synthesis was affordable even for rocket fuels. Since the better (exciplex) UV lamps are rather new, this field can only increase.
For rocket fuels, tertiary amines are better than hydrocarbons and usually easier to synthesize.
Of course, I want aza and diaza-cubane, methylated to be liquid. Obviously.
O yes: a fuel that ignites very quickly in liquid oxygen (trimethylgallium and -aluminium do that) but doesn't catch fire in air (these are pyrophoric). If any possible... Possibly easier, an oxidizer that lights "kerosene" (RP-1) by quickly contact but is harmless.
-----
Polymers of bicyclo[1.1.1]pentane and bicyclo[2.2.2]octane? The completely straight chains might be stiff and strong, possibly better than polyethylene, today's hi-tech fibre.
Dumb-looking alkanes like CCC(C)CCCC(C)CCCC(C)CCCC(C)CC could make a nice fluid for aeroplane hydraulics and lubricants, electronics coolant, transformer oil, vacuum grease, and so on, if produced in quantity.
Post by: OrgXemProf on December 14, 2014, 09:01:04 PM
In order to maximize density and Isp one must look to compounds that possess highly compact structures and contain considerable strain energy (think: Eaton & Cole's "cubane").
For many years the US Office of Naval Research funded an extensive program in chemical propulsion. An edited treatise has appeared that summarized progress that accrued in this program up to ca. year 2000 (vide infra).
Although now somewhat dated, individual articles presented the following publication nevertheless afford insight into what it takes to make a noteworthy rocket (or airbreathing missile) fuel:
Roy, G. D., Editor, "Advances in Chemical Propulsion", CRC Press:Boca Raton, FL, 2001. This publication is available online and can be downloaded as a pdf file c/o
http://cnqzu.com/library/Anarchy%20Folder/Rocketry/Propellants%20and%20Fuels/Advances%20in%20Chemical%20Propulsion.pdf
Post by: Shadow on December 15, 2014, 02:13:37 PM
Post by: OrgXemProf on December 15, 2014, 10:28:58 PM
Post by: curiouscat on December 15, 2014, 10:57:42 PM
I've been thinking about a suitable synthetic target for Shadow to pursue. Perhaps he might consider the conversion shown in the attachment.
Catalyst = LSD
Post by: Enthalpy on December 18, 2014, 10:34:57 AM
Specific impulse, for rocket propellants, yes. And because hydrogen atoms are so efficient in a fuel, any added -C bond must add very much strain energy to justify itself. Cyclobutanes bring a little bit, cyclopropanes are interesting, and odd strained compounds get interesting as compared with so-called "kerosene" (RP-1) - but provided they're safe and cheap enough and so on, that's why we still have kerosene.
No miracle launcher fuel has spread since decades, because most are too dangerous, or perform worse than kerosene, or cost too much - and the next step is hydrogen, for which engines exist already. So what can improve is (1) safety, especially by replacing hydrazine (2) liquid range, to take-off from Mars (3) maybe perhaps the performance.
By the way, how stable is bicyclobutane?
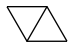
I've only found "enough to be isolated", which isn't accurate enough to pour 200t in a rocket... Even nitromethane (seducing as a monopropellant) is excluded as it detonates after a 100m fall. So is bicyclobutane stable at shocks, like a drop on concrete from 100m? At heat, say +200°C? Because, if a fuel can't cool the engine by flowing in the jacket, then we can burn ethylene, which is available and extremely efficient.
One difficulty with "energetic materials" is that the name mixes different uses that have opposite requirements. Explosives have the oxidizer in the same molecule, cruise missiles want volume-effective fuels burnt with air producing a small signature, liquid launchers want safe mass-effective fuels burnt with oxygen and care very little about density. Most proposed launcher fuels, with oxygen atoms, multiple bonds, or unstrained cycles, pursue wrong objectives.
Among the very few fuels that might be useable in liquid rockets and outperform kerosene and methane or cyclopropane are some cubane derivatives if mass-produced, some spiro compounds, maybe stellane (how efficient?)
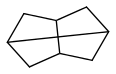
widely described in
http://www.tdr.cesca.es/TESIS_UB/AVAILABLE/TDX-0618107-110904/CAR_Tesi.pdf
ladderanes are no miracle
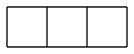
diasterane and triasterane would probably be good if mass-produced
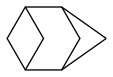
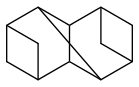
bicyclo[1.1.1]pentane is efficient and said to be stable
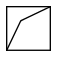
housane is efficient
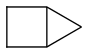
Most of these (not exhaustive list!) should rather be coupled in pairs or trios (but this wastes performance) to be liquid, and tertiary amine compounds are welcome.
The amine option has been investigated too little up to now. Aza brings performance and eases syntheses. Photosynthesis as well, enabled by the now available excimer lamps, whose purchase and electricity costs are affordable by a launcher, should be investigated.