Post by: Dan on December 27, 2014, 08:24:38 AM
Post by: kriggy on December 27, 2014, 03:43:19 PM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2F4poywAV.png&hash=e7fd845e310fcd2b57eddad8aba1b2b08e1a2f90)
Hope I didnt forget something obviously important :D
Im not realy sure about the acetal I saw it somewhere that it protects the keton I want and not the conjugated one. The 2nd thing is that Im not sure if NaOH is strong enough to deprotonate the nitrohexane. But another base could be used. The pyrrolidine formation should proceed easily as the nitro group is reduced to amino since its intermolecular reaction. Shiff base is reduced by the hydrogen present in the reaction mixture.
Post by: Altered State on December 29, 2014, 06:29:33 AM
Haha ok lets hit it :)
http://i.imgur.com/4poywAV.png
May I suggest another initial route?
It seems kind of strange to me using those starting materials :P
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FN0vbwNs.jpg&hash=18ee3526ff577264a03e715c50f6e517828869b3)
In this case you don't have to worry about the selectivity of the protection anymore, a few less steps (I think?) and less redox reactions (I like routes with less redox steps possible)
I cannot be sure about stereochemistry tho
Post by: kriggy on December 29, 2014, 07:00:49 AM
Damn those lithium compounds, I always forget that they exist :D
THe thing is that I saw synthesis of very similar compound in "Disconection approach" few days ago so I didnt want to just copy their synthesis since that would be useless for me to learn something (but I still used the nitro->amino-> reductive amination pathway which I realy like)
Post by: Altered State on December 29, 2014, 08:14:23 AM
Yea it was strange but thats why we are here :)
True that. Even though there are better or worse methods, what is amazing of organic synthesis is the many approaches one can take!
(but I still used the nitro->amino-> reductive amination pathway which I realy like)
I like it very much too. I would like to know about the yields of those cyclations
Post by: kriggy on December 29, 2014, 10:38:58 AM
You can find more on page 58 of "disconection approach" or in articles they quote:
I like it very much too. I would like to know about the yields of those cyclations
R.V.Stevens, A.W.M. Lee, J.Chem.Soc., Chem. Commun., 1982,102
R.V.Stevens, Acc. CHem.Res., 1984, 17, 289
I didnt read those articles but disconection approach says 68 for the second cyclizaton but the compound is bit different (one ring is 6 membered and the alkyl chain is shorter)
Post by: critzz on December 29, 2014, 10:49:37 AM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi59.tinypic.com%2Fmikj2u.gif&hash=f44e5f1bef768e2ef559b210dd1934a644381836)
Post by: kriggy on December 29, 2014, 11:33:43 AM
Post by: critzz on December 29, 2014, 12:18:12 PM
Post by: Dan on December 29, 2014, 12:53:27 PM
Can you comment on the diastereoselectivity (facial selectivity) of the hydrogenation? I would expect addition of hydrogen predominantly from the least hindered face, which would give the wrong diastereoisomer (see blow). I might be totally wrong though.
Post by: critzz on December 29, 2014, 03:51:08 PM
I guess both diastereomers are formed, rendering this synthesis useless (at least I tried).
However, I might have found something interesting about these pyrrole reductions?
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-4-3
Especially scheme 7, 9 and the end conclusion.
Post by: kriggy on December 29, 2014, 04:21:57 PM
Post by: phth on December 30, 2014, 08:58:38 PM
Post by: Altered State on December 31, 2014, 05:00:06 AM
Great job. Liked it.
Post by: kriggy on December 31, 2014, 06:06:09 AM
Post by: phth on December 31, 2014, 06:53:19 AM
Post by: SinkingTako on January 01, 2015, 12:37:49 AM
Also, can anyone recommend a good book on learning strerochemical control?
Post by: phth on January 01, 2015, 11:16:45 PM
http://www.amazon.com/exec/obidos/ASIN/0199270295/organischeche-20#customerReviews
http://www.organic-chemistry.org/books/reviews/0198503466.shtm
But here is a great resource with editorial reviews including books of all difficulties:
http://www.organic-chemistry.org/books/navi/organicchemistry.shtm
Post by: Dan on January 02, 2015, 07:28:41 AM
1. You seem to have gained a C in the rearrangement. I think you need an extra methylene in the starting material - so you go via a [4.2.0] bicyclic aminal.
2. Can you propose a synthesis of the starting material (so that the route starts from commercially available material)?
Post by: phth on January 02, 2015, 09:22:00 PM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FtKM9Uhb.png&hash=ed0a571788243492fbf040a93760d5cb56a84e64)
Post by: SinkingTako on January 03, 2015, 09:03:37 AM
Who's winner? let's get OP to decide! :)
Post by: critzz on January 03, 2015, 11:17:08 AM
What does a typical electrocyclic reaction procedure with hν look like (reaction conditions etc.)?
Post by: kriggy on January 03, 2015, 12:16:30 PM
This week there is a tie. Both critzz and phth have 8 steps (kriggy comes very close!).
Who's winner? let's get OP to decide! :)
Its not ONLY about number of steps. Having more steps that are reliable is better than fewer but unreliable IMO.
I realy like phth´s pathway. Its very creative
Post by: Altered State on January 03, 2015, 01:19:24 PM
I liked phth pathway too.
Looking forward to see what is the next challenge. I hope I have time to go for a route this time, I'm really busy these days, and Christmas celebrations don't help :P
Post by: Dan on January 04, 2015, 09:25:28 AM
I am inclined to go with phth's proposal as the winning entry. I like the creativity of the approach. I would like to see a synthesis of the proposed starting chiral acid chloride though...
I will share with you my own suggestion. I spent some time trying to come up with a chiral pool synthesis from pyrogluctamic acid, but failed to find something good. Eventually I settled on this as the shortest route. It is an enantioselective synthesis using Noyori asymmetric hydrogenation for stereocontrol. Not very inventive, but it is short.
1. Double addition of of lithiated hexyne to succinic anhydride at low temp (diketone obtained on aqueous workup)
2. Lindlar reduction [Edit: Alkenes formed in this step should be Z, not E]
3. Double Noyori reduction (the chirality sense at both asymmetric centres is the same)
4. Double tosylation
5. Double SN2 displacement by the chiral allylic amine (itself prepared in 3 steps from 3-octen-2-one via Noyori chemistry).
6. Grubbs metathesis
7. Alkene hydrogenation
Longest linear sequence is 7 steps.
Post by: orgopete on January 04, 2015, 09:51:48 AM
1. Double addition of of lithiated hexyne to succinic anhydride at low temp (diketone obtained on aqueous workup)
2. Lindlar reduction
I thought the step 1) intermediate would be a keto-carboxylate. If so second equiv to add to ketone, no?
Step 2), trans?
Post by: Dan on January 04, 2015, 10:29:13 AM
1. Double addition of of lithiated hexyne to succinic anhydride at low temp (diketone obtained on aqueous workup)
2. Lindlar reduction
I thought the step 1) intermediate would be a keto-carboxylate. If so second equiv to add to ketone, no?
I was hoping that at low T the tetrahedral intermediate would be stable enough to do this:
i.e.
Stable at low T?
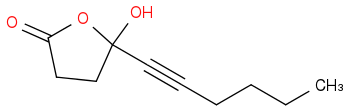 :rarrow:
:rarrow: 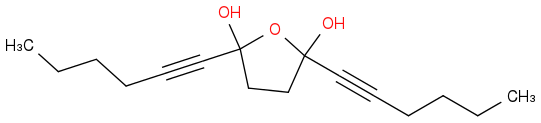
[but with OLi instead of OH - smiles won't let me have Li...]
I don't have journal access at the moment so I can't check precedent until next week...
Quote
Step 2), trans?
Oops. Of course Lindlar will give Z-alkenes - though this should not be a problem given their ultimate fate.
Alternative reduction methods (e.g. Cr(II) salts Link (https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-1986-31616)) could be used to get the E-enones shown.
Post by: Altered State on January 05, 2015, 03:57:22 PM
What do you think about this? http://pubs.acs.org/JACSbeta/jvi/images/issue29/ol403350e.jpg
Post by: kriggy on January 05, 2015, 04:19:14 PM
Shouldn't we propose a new problem? It's been a few days from Saturday.
What do you think about this? http://pubs.acs.org/JACSbeta/jvi/images/issue29/ol403350e.jpg
Seems to me that someone has too much free time :P
(or are you looking for ideas how to start total synthesis ;D )
Post by: Altered State on January 05, 2015, 06:46:51 PM
Shouldn't we propose a new problem? It's been a few days from Saturday.
What do you think about this? http://pubs.acs.org/JACSbeta/jvi/images/issue29/ol403350e.jpg
Seems to me that someone has too much free time :P
(or are you looking for ideas how to start total synthesis ;D )
A bit of free time after Christmas :P
I don't really care about the molecule, but the hung is that this is supposed to be a weekly thing, if we don't stock to that, it will probably die again... I've experienced it similar (though unrelated) threads in other forums.
Post by: kriggy on January 06, 2015, 03:59:57 AM
Post by: phth on January 06, 2015, 05:10:52 AM