Post by: jhonny3 on July 02, 2015, 08:29:36 PM
1-iodo-but-2-ene reacts with sodium methoxide to form what product? (or, in structural terms
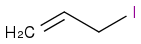 + NaOCH3). I think that the methoxide group will attack the beta carbon's hydrogen in an acid-base form and cause the elimination of the iodine, creating CH2CCH2. He thinks it will undergo an SN2 reaction and form
+ NaOCH3). I think that the methoxide group will attack the beta carbon's hydrogen in an acid-base form and cause the elimination of the iodine, creating CH2CCH2. He thinks it will undergo an SN2 reaction and form 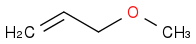 . The problem is that his feels more right, but I don't have a reason, as I have always been told that acid-base chemistry happens the fastest of all. Help?
. The problem is that his feels more right, but I don't have a reason, as I have always been told that acid-base chemistry happens the fastest of all. Help?
Post by: Unco on July 03, 2015, 01:59:54 AM
Post by: mjc123 on July 03, 2015, 04:35:03 AM
Post by: spirochete on July 05, 2015, 08:06:11 PM
Allenes are pretty unstable generally, so usually allene formation will not be the answer for problems like this. More generally, primary halides tend to favor substitution even with fairly strong bases like methoxide. So the ether is a better answer.
You are correct that acid base reactions tend to go very quickly, but the reaction you're describing to give the allene is not a simple proton transfer, it is an E2 reaction. E2 reactions have a much higher barrier than typical proton transfers, at the very least for entropic reasons. E2 reactions also have other considerations that simple proton transfers don't have, such as the preference for an anti peri planar conformation.