Post by: Enthalpy on May 15, 2016, 06:41:22 AM
The nicely strained and stable bicyclo[1.1.1]pentane and oligomers are synthesized from [1.1.1]propellane usually, by breaking the central bond.
https://en.wikipedia.org/wiki/1.1.1-Propellane
[1.1.1]propellane is obtained from gem-di(chloromethyl)ethene (aka 3-chloro-2-chloromethyl-1-propene), on which dibromocarbene cyclopropanates the double bond, and then methyllithium closes efficiently two more cyclopropane cycles between the carbons that bear bromine and chlorine.
http://www.orgsyn.org/demo.aspx?prep=V75P0098
(Drawing appended here)
The cyclopropanation takes 4 days, a definite drawback for a product to be torched in hundreds of tons.
http://www.chemicalforums.com/index.php?topic=79637.msg290422#msg290422
- Do the chlorines slow down the cyclopropanation? From paragraph 29.2.1.1.6.1 in Houben-Weyl's Science of Synthesis (Google book): "Electron-deficient alkenes are less reactive towards dichlorocarbene"
- Do you believe the chlorines and bromines can be swapped in the synthesis as in the drawing's lower part? This has other potential advantages.
- Would the dibromoalkene react more quickly with the dichlorocarbene?
Post by: discodermolide on May 15, 2016, 12:36:42 PM
Post by: Enthalpy on May 15, 2016, 03:32:08 PM
The reference synthesis takes pentarythritol, converts three hydroxyls to chlorides, the fourth to an acid, then eliminates by heat 1*C, 2*O, 1*H, 1*Cl
http://www.orgsyn.org/demo.aspx?prep=V75P0089
http://www.dtic.mil/dtic/tr/fulltext/u2/a267508.pdf
Drawing appended.
----------
Orgsyn calls direct halogenation the "best alternative": 34% yield (starting from the monochloro), mixed products difficult to separate. Though, I feel it interesting for mass production:
- Isobutene and chlorine are really cheap, even for a bad yield. Bromine less so (4k$/t), but it can be recovered from the waste.
- The reaction must be swift and low-tech. Excellent GaN 405nm Leds dissociate Br2, excellent AlGaN 385nm Leds and mp-Hg lamps dissociate Cl2.
- Accurate distillation is a small worry at big scale, and freezing instead must separate the dihalo isomers better.
- Addition to the double bond is hence limited. Little halogen and much light are said to help. This is easily done in a continuous process. Cl2 molecules absorb 365nm light with 4*10-20cm2, or over 10mm at 1kPa and 300K, while Br2 absorbs 15 times better at 405nm and other species nothing.
- Allylic substitution is very selective over vinylic, and other reactions are visibly under control.
- Precipitation of the dihalo may already limit the number of halogenations in the reported yield. This improves if the reactor distills the species (drawing appended), so that only dihalo exits.
- Steric hindrance may already hamper the gem-dichloro, offsetting the bond dissociation energy that favours it. Bromine would improve both the hindrance and the bond dissociation energy.
Taking ethane as a model available in "Bond dissociation energies" from Yu-ran Luo
http://staff.ustc.edu.cn/~luo971/2010-91-CRC-BDEs-Tables.pdf
we see that a first chlorine makes a second hydrogen abstraction easier locally, while a first bromine brings no clear preference.
BDE +-
-------------------------
420,5 1,3 CH3CH2-H
-------------------------
423,1 2,4 CH2ClCH2-H
406,6 1,5 CH3CHCl-H
397,9 5,0 CH3CHCl2-H
-------------------------
415,1 8,4 CH2BrCH2-H
415,0 2,7 CH3CHBr-H
397,1 5,0 CH3CBr2-H
-------------------------
Would you see better tricks? NBS and NCS are known for allylic halogenation, but as they allegedly create X2 first, steric hindrance should stay the same. Hypohalite acids? X-O-tBut? HX elimination bringing the double bond?
Comments, remarks, suggestions, even objections maybe? Thank you!
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on May 16, 2016, 09:20:58 AM
I think the alternative route may be too expensive.Thanks Discodermolide!
What makes dichlorocarbene-on-dibromobutene more costly than dibromocarbene-on-dichlorobutene: the reactants and their amounts, the lower yields, the slower reactions, the risks...?
If a launcher decides some day to consume every month 200t of the exotic stuff, I imagine it would be produced at a refinery, in an especially built annex, where byproducts like LiCl, LiBr, HBr... would be recycled into the LiCH3, Br2, HOBr etc used by the process. Would that make sense for these quantities already?
Post by: discodermolide on May 16, 2016, 09:39:57 AM
Thus dichlorocarbene plus dichlorobutene may be cheaper. At industrial scale it may also be safer. The risks of carbene chemistry are large, very concentrated NaOH, dihalocarbenes, easily polymerised olefins, etc.
My option would be to consider an industrial flow system for this chemistry rather than batch.
Post by: Enthalpy on May 16, 2016, 04:11:19 PM
On pages 2-5 (Pdf 8-11) of his PhD thesis
http://thesis.library.caltech.edu/3853/1/Rosen_aj_1964.pdf
Allan Joseph Rosen cites several groups who got 55-67% of chloromethyl instead of ring opening, and up to 16 times more chloromethylcyclopropane than chloroalkene through vapour-phase photochlorination by Renk et al.
Apparently, bromine uses to open the ring, hence better chlorine. Byproduced HCl is said to harm and should be removed. What do prior bromines do and does the second chlorination work? Opinions welcome!
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on May 16, 2016, 04:54:15 PM
My gut feeling is that bromo compounds are more expensive than chloro.
Thus dichlorocarbene plus dichlorobutene may be cheaper. At industrial scale it may also be safer. The risks of carbene chemistry are large, very concentrated NaOH, dihalocarbenes, easily polymerised olefins, etc.
My option would be to consider an industrial flow system for this chemistry rather than batch.
If chlorine everywhere is possible, fantastic! But does methyllithium couple two carbons that wear chlorines? I've read it between bromine and chlorine up to now, and once with 1,3-dibromopropane. The last step of the reference synthesis, with 80% yield from dibromodichloro to propellane, suggests that methyllithium never couples chlorine-bearing carbons.
Flow rather than batch permits to draw the intermediates (especially from halogenations) before they react too much, to keep halogen proportions low... many advantages. But if the cyclopropanation takes 4 days, flow won't help: to produce 200t in a month, it would need >27t in the reactor, ouch. Hence a fast reaction is desired.
Post by: discodermolide on May 16, 2016, 10:24:39 PM
Also a transition metal catalysed reaction may be possible for the propellane forming step.
Post by: Enthalpy on May 18, 2016, 02:21:18 AM
The last intermediate to propellane would be 1,1,1-tribromo-2,2,2-tri(chloromethyl)ethane, tagged 23 on the appended drawing.
The suggested steps to 23, from isobutene (even more correctly represented now) and acetic acid, are most probably nonsense. Please consider them as an attempt to trigger off better ideas.
Marc Schaefer, aka Enthalpy
Post by: wildfyr on May 18, 2016, 09:26:08 AM
Post by: discodermolide on May 18, 2016, 10:51:14 AM
Post by: wildfyr on May 19, 2016, 10:59:16 AM
Post by: discodermolide on May 19, 2016, 11:48:41 AM
Post by: Enthalpy on May 20, 2016, 06:06:15 AM
Meanwhile I have doubts that the Cl and Br can be swapped before the cyclization to propellane as I had hoped in the first message. CH3Li can also close cycles between two Br-bearing carbons, so a condition may be that all Br are vicinal and only the Cl remote. Opinions?
Which chlorinating agent and condition would you suggest to get di(chloromethyl)ethene from butene, instead of addition products or geminal dichloro? For instance, photochlorination of 1,1-dichloroethane gives 80-90% 1,1,1-trichloroethane industrially, probably the wrong way here. HClO, t-ButOCl, NCS, other?
Alternately, could bromochloromethane add to allyl chloride to give gem-di(chloromethyl)ethene after HBr abstraction, as on the appended sketch? The bromine is hopefully more reactive than the allylic chlorine, avoiding a dimer.
It would take reactants available at a vinylchloride or epichlorihydrin factory and use similar processes, for instance at an annex there.
If gem-di(chloromethyl)ethene is easy enough to produce, the polymer is worth a try too. And compound 24 might lead to 23 as well.
Marc Schaefer, aka Enthalpy
Post by: kriggy on May 21, 2016, 04:35:41 AM
maybe ?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FFmKDVtD.png&hash=3b0ff1a765abea780c393f0706ccd8271d24fe72)
Knoveagel followed by Hunsdiecker ? I mean those reactions work well so it might be better solution that looking for fancy halogenations
Post by: Enthalpy on May 22, 2016, 07:37:24 PM
Up to now I've looked at isobutene, propene, chlorine... because they're cheap, that's the only reason. 1M€/200t would be a reasonable selling price for the oligomerized propellane, so every reactant must be dirt-cheap or recycled (like NCS can be if I read properly).
Malonic acid would be obtained from dimethyl malonate, itself from dimethoxymethane obtained from formaldehyde and methanol, is that it? Or by "fermentation of glucose" as wiki tells? On Alibaba I see malonic acid around 7$/kg in ton amount, that is 13$/kg of isobutene. Maybe production at the plant can improve that?
I suppose AgCl from Hunsdiecker is completely recycled.
----------
I have a small doubt about the number of remaining carbons after Hunsdiecker.
Post by: kriggy on May 23, 2016, 04:26:45 AM
I have a small doubt about the number of remaining carbons after Hunsdiecker.
Crap right, hunsdiecker decarboxylats so there is one less carbon for each carboxylate. :-[
What one could do is do the knovenagel with diethylmalonate, then reduce the ester to alcohol and then halogenate but one more steps.
Anyway, you dont have to use silver salt for the Hunsdiecker reaction. Friend of mine did that few weeks ago, just acid and NBS or acid, sodium acetate and NBS. I suppose that it would be very similar with NCS.
Post by: Enthalpy on May 23, 2016, 01:50:04 PM
Is that what you suggest?
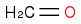 +
+ 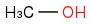 :rarrow:
:rarrow: 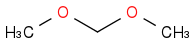 :rarrow:
:rarrow:  :rarrow:
:rarrow:  :rarrow:
:rarrow: 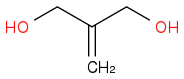 :rarrow:
:rarrow: 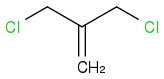
Does the reduction need LiAlH4? I see prices like 200$/kg on Alibaba in ton amount.
Or would alcohol or chlorine suffice for Knoevenagel? Propane-1,3-diol getting a =CH2 at the middle.
Or eliminate CO2 by heat somehow? Possibly with the chlorine already in place?
Post by: kriggy on May 25, 2016, 07:02:38 AM
Im not sure about the reduction, I did it with NaBH4+MeOH but there might be another, cheper systems. maybe even hydrogen reduction could work?
I dont think that alcohol/chlorine would work for Knovennagel, the CH2 is activated by hyperconjugation and none OH or Cl are hypercojugating groups
Post by: Enthalpy on May 25, 2016, 12:25:31 PM
To reduct an ester to an alcohol, I've found the Bouveault–Blanc reduction:
https://en.wikipedia.org/wiki/Bouveault-Blanc_reduction (animation on Spanish and Portuguese pages)
http://www.orgsyn.org/demo.aspx?prep=cv3p0671
with just Na in ethanol. Very promising, isn't it? If the two functions per molecule don't interact in between.
Post by: Enthalpy on May 26, 2016, 03:11:19 PM
CHCl=CH2 + CH2BrCl :rarrow: CH2Cl-CH(CH2Cl)-CH2Br
Addition of dichloromethane on alkene has been observed but as anti-Markovnikov
https://www.jstage.jst.go.jp/article/cl1972/7/4/7_4_367/_pdf
out.
My present impression is that the gaseous chlorination of isobutene is the cheapest route, as the reactants are dirt-cheap and the conditions just heat, but will produce gem-dichloro essentially because this radical is easier. So:
would you know chlorinating agents, catalysts, reaction conditions that chlorinate the distant allylic carbons of isobutene preferentially? Possibly a bulky molecule hindered by the first chlorine atom?
NCS seems cheap to regenerate but, said to create Cl2, it shouldn't show a different selectivity.
HClO? t-ButOCl? Other?
Post by: Enthalpy on May 27, 2016, 07:23:20 PM
The GDR patent 106345 reacts gases to obtain 1/3 of the desired dichloro: drawing appended. Cited by the appended US pat 4587367.
The distribution of the products contradicts the radical stability: chlorinated vinyl position, no gem dichloro... Either it's baloney, or the hydrogens move often.
Starting instead with 3-chloro-2-methylpropene gives the same distribution of products, suggesting it's the first step when starting with isobutene.
The US pat 4587367 chlorinates instead with SO2Cl2 and a catalyst to obtain again the same distribution of products. SO2Cl2 is less volatile but is regenerated by guess what.
So the simplest way looks as good as the others and, despite limited yield, really cheap.
Post by: Enthalpy on June 05, 2016, 09:40:51 AM
- Does Br substitute to OH rather than Cl in 20?
- Does Zn insert at Br rather than Cl?
- Does BrZnR couple with CBr4 rather than at the chlorines of R?
- Is Zn a sensible choice, or are others (Li, Mg, B, Sn...) better, especially safer?
I had hoped to couple through HX elimination, but due to the halogens, all hydrogens seem equivalent: at CHBr3 and the primary and the tertiary at trichlorobutane.
Thank you!
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on June 09, 2016, 06:58:59 PM
Post by: kriggy on June 10, 2016, 12:55:35 AM
a) I would say yes but depends on conditions http://www.organic-chemistry.org/namedreactions/appel-reaction.shtm
b) Not sure here, it works for grignards so might be the same with Zn
c) No idea but bromines are AFAIK more reactive in coupling reactions than chlorines
d) Well it depends, but I wouldnt want to work with organotlithium compounds in multitonne scalef or example if it could be avoided
Post by: Enthalpy on June 14, 2016, 06:46:52 AM
Well, methyllithium is the only good path to propellane I've seen on the Web. The good side is that in 1h it's finished, but for 200t/month 24/7 the reacting amount is still about 300kg.
I wanted to avoid the 4-days-long cyclopropanation, but it looks rather harmless, and the recent alternative I suggested needs activated metals, so I doubt it's any better.
Opinions please?
Post by: Enthalpy on June 17, 2016, 09:58:45 AM
35.1.1.1.2 of "Science of Synthesis" by Houben-Weyl
which tells that the gem-dichloro is half as abundent as chloros spread across the chain.
If someone knows a chlorinating agent that disfavours the gem-dichloro even more, please tell!
tBuOCl?
This makes the hypothetic alternative path of May 16, 2016 more credible (appended again, isobutene corrected).