Post by: Bidagdha_TADIR on October 25, 2016, 07:59:26 AM
NO2
|
Cl---C----Br
|
ONO
[Sorry, but I couldn't attach the structure from chemdraw for some reason].
Post by: sjb on October 25, 2016, 09:01:59 AM
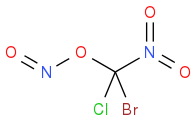 or
or 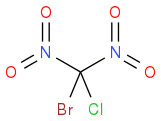 ? What are your thoughts? (Just need to check the structure as sometimes spacing throws these off)
? What are your thoughts? (Just need to check the structure as sometimes spacing throws these off)
Post by: Bidagdha_TADIR on October 25, 2016, 12:34:31 PM
By the way, How did you upload it? I couldn't do it by using 'insert image'.
Post by: AWK on October 25, 2016, 12:52:17 PM
Quote
By the way, How did you upload it? I couldn't do it by using 'insert image'.Just press quote on sjb post, and you can see SMILES codes for both structures. Such codes are converted for nice pictures on our forum.
Post by: orgopete on October 25, 2016, 06:11:47 PM
Post by: lhpVietNam on November 01, 2016, 10:33:00 AM
Post by: Bidagdha_TADIR on November 04, 2016, 08:00:42 AM