Post by: Nekromantis on November 09, 2016, 04:09:42 AM
Sorry, for my english :)
Post by: sjb on November 09, 2016, 04:32:04 AM
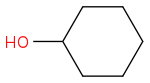 + e.g.
+ e.g. 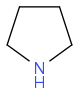 to
to 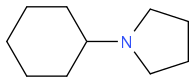 ? How did it fail, what did you get back? I'd think about reductive animation, perhaps; or some of the hydrogen borrowing reactions by Williams et al (amongst others)
? How did it fail, what did you get back? I'd think about reductive animation, perhaps; or some of the hydrogen borrowing reactions by Williams et al (amongst others)
Post by: Nekromantis on November 09, 2016, 04:39:12 AM
Post by: sjb on November 09, 2016, 06:13:46 AM
Post by: Dan on November 09, 2016, 07:44:22 AM
NMR analysis showed mixture of alcohol and amine.
Which amine - tert amine product or secondary amine starting material?
If you see cyclohexanol in the mixture, that indicates competitive (or preferential) hydrolysis. Performing the reaction under anhydrous conditions should prevent it.
As has been suggested, reductive amination is usually the preferred method for this type of transformation.
Post by: Nekromantis on November 09, 2016, 08:09:08 AM
NMR analysis showed mixture of alcohol and amine.
Which amine - tert amine product or secondary amine starting material?
If you see cyclohexanol in the mixture, that indicates competitive (or preferential) hydrolysis. Performing the reaction under anhydrous conditions should prevent it.
As has been suggested, reductive amination is usually the preferred method for this type of transformation.
NMR showed alcohol and secondary amine - pyrrolidine or piperidine... i thought about amination but catalysts using in this reaction are very expensive and i dont have guarantee that this reaction will be positive.
Post by: orgopete on November 09, 2016, 08:55:42 AM
Post by: Dan on November 09, 2016, 12:44:19 PM
catalysts using in this reaction are very expensive and i dont have guarantee that this reaction will be positive.
10% Pd/C is not so expensive, 1 g is cheap enough to buy for a test even if you are worried it won't work, certainly cheaper than spending your time running lots of reactions with 0% yield.
Post by: Nekromantis on November 09, 2016, 01:02:47 PM
catalysts using in this reaction are very expensive and i dont have guarantee that this reaction will be positive.
10% Pd/C is not so expensive, 1 g is cheap enough to buy for a test even if you are worried it won't work, certainly cheaper than spending your time running lots of reactions with 0% yield.
1g cost about 30$ but I need 50-100 g final compound to make all physicochemistry and reactions.
Maybe Grignard reaction between cycliccompound-MgBr and amine? Convert -OH to Cl eg. SO2Cl, next step reaction with Mg and finaly with pyrrolidine?
Post by: Dan on November 10, 2016, 02:18:46 AM
1g cost about 30$ but I need 50-100 g final compound to make all physicochemistry and reactions.
Yes, but it shortens your synthesis to 1 step and the reaction is usually so clean that very minimal if any purification is necessary. I appreciate that Pd/C is not the cheapest reagent in the world, but if you cost all the reagents and labour time required for a messy alcohol activation/SN2 procedure, you might be surprised.
Quote
Maybe Grignard reaction between cycliccompound-MgBr and amine?
What is the mechanism for that transformation?
Post by: Nekromantis on November 10, 2016, 04:24:26 AM
1g cost about 30$ but I need 50-100 g final compound to make all physicochemistry and reactions.
Yes, but it shortens your synthesis to 1 step and the reaction is usually so clean that very minimal if any purification is necessary. I appreciate that Pd/C is not the cheapest reagent in the world, but if you cost all the reagents and labour time required for a messy alcohol activation/SN2 procedure, you might be surprised.
Could you give me a some leads for article where its using Pd/Cl ?
Quote
Maybe Grignard reaction between cycliccompound-MgBr and amine?
What is the mechanism for that transformation?
[/quote]
I read, that this reactions fail. I thought that, MgBr could react with N atom in amine... I have (-)-menthol and I transformed -OH gropu to Cl, but reaction between this Cl and amine dont work... Why? I did many PTC reaction, N-alkilation and result was great. But in menthol don't want react.
Post by: Dan on November 10, 2016, 05:54:42 AM
Could you give me a some leads for article where its using Pd/Cl ?
I had a quick look and actually it seems catalytic reductive amination of cyclohexanone with secondary amines and H2 is much more challenging than I thought - it is usually performed at high temp and pressure, so maybe not suitable for you. Sorry about that. There are lots of other methods though, see here for leads:
http://www.organic-chemistry.org/synthesis/C1N/amines/reductiveamination.shtm
Post by: Doc Oc on November 10, 2016, 11:55:03 AM
Post by: AWK on November 10, 2016, 04:02:26 PM
Post by: kriggy on November 11, 2016, 01:26:03 AM
And of course, then there is the famous Buchwald-Hartwig or Chan-Lam (If you can get the boronic acid easily)
Post by: Nekromantis on November 12, 2016, 07:25:22 AM
Cl is a very bad leaving group. Better options would be to convert it to a Br or I, or more simply to mesylate or tosylate the alcohol (which you suggested the the p-TsCl). Dissolve that in DMF, add some K2CO3 and the amine. Heat may be necessary to facilitate reaction, but I've done lots of amine alkylations this way. Cl has never worked, I've always needed to swap it for a more labile leaving group.
In monday i will try make a reaction with p-toluenosulfonyl chloride. I found reaction where they used alcohol with p-toluenosulfonych chloride. Next step was dissolving imidazole in DMF and adding sodium. The heating reaction by 1 hour and they adding transformed alcohol. Byt result this reaction was not succesfull only 29%, but maybe this reaction with K2CO3 with cyclic amine eg. pyrrolidine will be better.
Quote
http://dergipark.ulakbim.gov.tr/tbtkchem/article/viewFile/5000024385/5000024622
This link is not working
Post by: AWK on November 15, 2016, 07:59:44 AM
try alao
http://journals.tubitak.gov.tr/chem/issues/kim-10-34-2/kim-34-2-16-0903-46.pdf
or
10.3906/kim-0903-46 (DOI the second link in google search)
Post by: Nekromantis on November 21, 2016, 07:15:00 AM
But now I must clean the product.
1-(1S)-Menthylimidazole (1b):
To a solution of imidazole (13.2 g, 194 mmol) in dry N,N-dimethylformamide (DMF, ca. 120 mL), sodium
hydride (5.41 g, 225 mmol) was added portionwise at 0 °C under a nitrogen atmosphere. The mixture was stirred for 15 min at the same temperature, and then (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl-p-toluenesulfonate (1a) (20.0 g, 64.4 mmol) was added in one portion to the reaction mixture under an inert atmosphere. The reaction mixture was warmed to room temperature and then heated to reflux for 24 h, after which it was poured into cold water (ca. 300 mL). The crude product was extracted with ethyl acetate (ca. 3 200 mL). The organic layers were collected and repeatedly washed with water (ca. 5 50 mL) followed by a saturated sodium chloride solution (ca. 50 mL). The organic extract was finally dried
with anhydrous MgSO4 and filtered, and the volatiles were removed in vacuo. The crude product was purified by column chromatography (silica, petroleum ether/EtOAc 95:5–20:80) to give the product
(1b) as a white solid (3.83 g, 29%).
I think, that in first step I'll separate a precipitate (eg. unreacted K2CO3) from organic layer. Next I'll make all what was described in article.
What do you think?
Post by: orgopete on November 21, 2016, 07:17:16 PM
Today I made this reaction using K2CO3 in DMF, but i used pyrrolidine instead imidazole like was descriped in article: Eur. J. Inorg. Chem. 2015, 1604-1615.
What do you think?
Look at examples 17, 18, and 19 in the reference posted by AWK!
Post by: Nekromantis on November 28, 2016, 08:21:02 AM
1. 4-Methyl-1-(1-methylethyl)cyclohexene,
2. Menthol
3. Product
4. Like a double menthol
Amount of product is small about 20%. Very much is menthol. Maybe my product decomposed in feeder, so I have peak 1 and 2, or I will should reaction parameters. I wonder why I have menthol? I used pure Ment-O-pTOS.
Post by: rolnor on November 28, 2016, 09:28:38 AM