Post by: owk9688 on December 08, 2016, 09:55:31 PM
Would a reverse phase column help the 2 spots seperate better or is there a different way to get past this issue?
Thanks for any help
Post by: phth on December 08, 2016, 11:57:47 PM
Post by: MOTOBALL on December 09, 2016, 12:25:21 AM
Run the plate 4 or5 times, allowing to dry thoroughly between each run.
I have found this technique to work very well for partially substituted polyols.
Post by: Dan on December 09, 2016, 02:03:20 AM
acetone/hexane
ether/hexane
EtOAc/toluene
acetone/toluene
ether/toluene
EtOAc/(1:1 hexane/toluene)
acetone/(1:1 hexane/toluene)
ether/(1:1 hexane/toluene)
Post by: TheUnassuming on December 09, 2016, 03:09:14 PM
Reverse phase generally doesn't work great with very non-polar compounds.
Post by: wildfyr on December 11, 2016, 05:22:40 PM
Post by: owk9688 on December 11, 2016, 11:27:53 PM
Post by: phth on December 12, 2016, 01:13:04 PM
Post by: Babcock_Hall on December 12, 2016, 04:01:00 PM
@phth, What is an A/B extraction?
Post by: Dan on December 12, 2016, 04:09:08 PM
Alumina is worth a shot, though usually in my experience the Rf on alumina is higher than on silica.
Can you use a less greasy protecting group that will make purification easier?
Post by: wildfyr on December 12, 2016, 05:49:07 PM
Take your triphenol and SLLOOOWWLLY add 1 eq TMS-Cl in a dilute solution, then isolate the mono TMS (distillation perhaps? I'm sure someone has made it before and scifinder will find it). Then take this pdt, and add 2.1 eq of TBDMS-Cl (my preferred TBDMS protection conditions are DCM, 1 eq alcohol, 1.05 eq TBDMS-Cl, 2.1 eq imidazole, RT overnight. Has literally never failed. Ive also run it in THF, MeCN and DMF with no problems except the usual DMF extraction pain). Afterwards, do a water wash.
Boom, di-TBDMS protected triphenol. No column.
Maybe flash it with hexane through a small silica plug to push off TBDMS dimer that will form with the slight excess of TBDMS-Cl, then rinse your product through with DCM.
You might even be able to do this in one pot if you can get the first TMS protection done nicely.
I never thought TBDMS protection (which I did constantly for two years while working on SuFEx) would be so widely applicable on this forum.
Post by: wildfyr on December 13, 2016, 11:08:06 AM
Post by: phth on December 13, 2016, 11:34:07 PM
What about this guys:
Take your triphenol and SLLOOOWWLLY add 1 eq TMS-Cl in a dilute solution, then isolate the mono TMS (distillation perhaps? I'm sure someone has made it before and scifinder will find it). Then take this pdt, and add 2.1 eq of TBDMS-Cl (my preferred TBDMS protection conditions are DCM, 1 eq alcohol, 1.05 eq TBDMS-Cl, 2.1 eq imidazole, RT overnight. Has literally never failed. Ive also run it in THF, MeCN and DMF with no problems except the usual DMF extraction pain). Afterwards, do a water wash.
Boom, di-TBDMS protected triphenol. No column.
Maybe flash it with hexane through a small silica plug to push off TBDMS dimer that will form with the slight excess of TBDMS-Cl, then rinse your product through with DCM.
You might even be able to do this in one pot if you can get the first TMS protection done nicely.
I never thought TBDMS protection (which I did constantly for two years while working on SuFEx) would be so widely applicable on this forum.
Post by: Dan on December 14, 2016, 02:29:12 AM
If you protect two of the alcohols as an acetal/ketal, then you only need 1 protecting group step.
But phloroglucinol is:
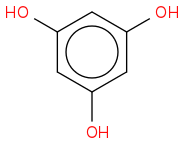
Initially when I saw "phloroglucinol" written, I assumed it was a sugar polyol and that an acetal/ketal strategy might be the answer, but it's not the case unfortunately.
Post by: Dan on December 14, 2016, 03:03:16 AM
What about this guys:
Take your triphenol and SLLOOOWWLLY add 1 eq TMS-Cl in a dilute solution, then isolate the mono TMS (distillation perhaps? I'm sure someone has made it before and scifinder will find it). Then take this pdt, and add 2.1 eq of TBDMS-Cl (my preferred TBDMS protection conditions are DCM, 1 eq alcohol, 1.05 eq TBDMS-Cl, 2.1 eq imidazole, RT overnight. Has literally never failed. Ive also run it in THF, MeCN and DMF with no problems except the usual DMF extraction pain). Afterwards, do a water wash.
Boom, di-TBDMS protected triphenol. No column.
Maybe flash it with hexane through a small silica plug to push off TBDMS dimer that will form with the slight excess of TBDMS-Cl, then rinse your product through with DCM.
You might even be able to do this in one pot if you can get the first TMS protection done nicely.
I never thought TBDMS protection (which I did constantly for two years while working on SuFEx) would be so widely applicable on this forum.
This strategy relies on the assumption that mono-TMS phloroglycinol is a lot less reactive than phloroglucinol. I would be surprised if the difference is that large. Maybe the strategy would work better with a more stable PG (orthogonal to TBS) that would survive chromatography, so even if the selectivity in the monoprotection step is low, at least the monoprotected product could be purified and isolated.
Presumably the next step in the synthesis is functionalisation of the OH of the diprotected triphenol. Another strategy would be to start from 3-hydroxy-5-iodophenol or a 3,5-dihydroxyphenylboronic acid derivative [though I have not checked commercial availability], whack on 2 x PG and then introduce the 3rd O substituent by cross coupling (i.e. Ullman or Chan-Lam type reaction). This could be a lot more direct than fiddling with a tricky PG pattern.
Post by: rolnor on December 14, 2016, 12:29:28 PM
Post by: wildfyr on December 14, 2016, 08:03:38 PM
Hmmm... what about monotosylating or mono acetylating phosphorglucinol? It should be a pretty powerful electron withdrawing group which might make the other alcohols less reactive like you're describing, and favor mono protection. the mono, bi, and tri tosyl or acetate might also be easier to separate by recrystallization or column if they occur than TBDMS. Then you can di-TBDMS protect, and base hydrolyze the tosyl or acetate. Aryl esters and sulfonate are already pretty base hydrolysis unstable, it shouldnt be too hard.
Post by: pgk on January 30, 2017, 12:49:50 PM
Post by: hypervalent_iodine on January 30, 2017, 04:49:57 PM
Addition of a drop of triethylamine per few ml of such a solvent, may help separating TLC spots that have similar Rf. Unfortunately, this is not reproductible.Ttriethylamine cannot be added in the chromatographic column because it destroys the contained silica or alumina.
Really? I didn't think adding low percentages of TEA (1-3 %) to columns was that uncommon. I myself have done it several times to assist in reducing streaking and improve separation of amines.
It is usually a good idea to pretreat plates with the TEA solvent before you run them, also.
Post by: wildfyr on January 30, 2017, 08:26:39 PM
Post by: hypervalent_iodine on January 30, 2017, 09:09:10 PM
Ive seen the TEA trick in columns in papers before, I dont think it hurts silica gel. You have to wash your product with aqueous acid to get it out afterwards though.
It deactivates the silica, which might be what was meant by destroys. However, deactivating it is the point of adding it, so I'm not sure I'd view it as a problem.
Correct about the acid wash, though it is also possible to vac it off if your compound is acid sensitive.
Post by: pgk on January 31, 2017, 09:34:17 AM
A little amount of triethylamine increases polarity that helps a better separation; but it simultaneously increases the dielectric constant of the solvent that might lead to partial and random coagulation of silica particles due to electrostatic forces.
Furthermore, triethylamine is rarely free of water traces and as a consequence, a little but significant amount of silica might be dissolved. Thus, a better separation might be achieved, but with moderate repeatability due to the random coagulation and sedimentation of silica particles in the column.
All above is not important, regarding TLC monitoring but it is quite important, regarding the validity of a scientific work and very important, regarding a patent’s validity.
Post by: hypervalent_iodine on January 31, 2017, 09:50:24 AM
The problem is a moderate repeatability.
A little amount of triethylamine increases polarity that helps a better separation; but it simultaneously increases the dielectric constant of the solvent that might lead to partial and random coagulation of silica particles due to electrostatic forces.
Furthermore, triethylamine is rarely free of water traces and as a consequence, a little but significant amount of silica might be dissolved. Thus, a better separation might be achieved, but with moderate repeatability due to the random coagulation and sedimentation of silica particles in the column.
All above is not important, regarding TLC monitoring but it is quite important, regarding the validity of a scientific work and very important, regarding a patent’s validity.
I wasn't aware of that, thank you for sharing. Working in the realm of patents is only something I have recently become involved in. Most of previous work where I had used TEA was in the context of the purification of an extremely recalcitrant and sensitive cyclic amine. Admittedly, TEA wasn't successful there (though it had been with compounds earlier in the sequence).
Post by: rolnor on January 31, 2017, 09:55:55 AM