Post by: mrspock on August 22, 2018, 11:28:44 AM
Post by: chenbeier on August 22, 2018, 12:49:07 PM
Post by: mrspock on August 22, 2018, 01:02:15 PM
Post by: Babcock_Hall on August 22, 2018, 05:22:38 PM
Post by: billnotgatez on August 22, 2018, 05:29:14 PM
What concentrations of
sulfuric acid and formic acid
do you intend on using.
H2SO4
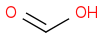
Post by: mrspock on August 22, 2018, 09:32:16 PM
Post by: hypervalent_iodine on August 22, 2018, 10:35:23 PM
two liters of sulfuric acid 95% and one liter of formic acid 85%
Is there a particular reason you need to do it on such a large scale?
Post by: mrspock on August 29, 2018, 04:01:44 PM
Post by: chenbeier on August 29, 2018, 04:12:57 PM