Post by: painterinmymind on February 13, 2019, 04:05:20 PM
(https://upload.wikimedia.org/wikipedia/commons/thumb/5/5c/4-Cyano-4%27-pentylbiphenyl.svg/613px-4-Cyano-4%27-pentylbiphenyl.svg.png)
If a CO2 were to be added between the two phenyl rings, what would the resultant chemical be called? Would this even be possible?
My aim is to suggest a synthetic route for both chemicals so I would assume reacting benzylnitrile with pentylbenzene could be a potential route, right? I'm not sure if benzylnitrile is a commercially available product however.
Post by: sjb on February 13, 2019, 04:21:56 PM
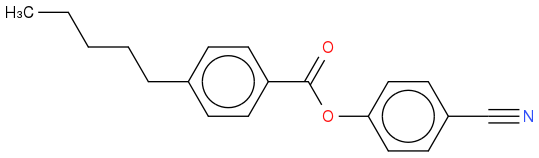 or maybe with the ester the other way around.
or maybe with the ester the other way around. Reaction of an appropriately substituted benzonitrile (benzyl nitrile would have an extra CH2 between the ring and the nitrile) with an appropriate pentylbenzene would give your initial compound - maybe with 4-cyanobenzeneboronic acid and 1-bromo-4-pentylbenzene in a Suzuki?
As to the ester - look into classical ways of generating these
Post by: painterinmymind on February 13, 2019, 05:05:55 PM
Do you mean something likeor maybe with the ester the other way around.
Reaction of an appropriately substituted benzonitrile (benzyl nitrile would have an extra CH2 between the ring and the nitrile) with an appropriate pentylbenzene would give your initial compound - maybe with 4-cyanobenzeneboronic acid and 1-bromo-4-pentylbenzene in a Suzuki?
As to the ester - look into classical ways of generating these
Yes precisely that! I assume similar initial compounds which are able to undergo esterfication would be used? Perhaps if there was a carboxyl group on the benzonitrile and an alcohol group on the pentylbenzene for example (I'll probably have to look into suitable reactants).
Thank you for the help though. Really helpful and much appreciated.
Post by: wildfyr on February 13, 2019, 08:18:12 PM
Post by: careerorbits on March 27, 2019, 04:29:46 AM