Hello
I found an exercise that asked me to assign "true" or "false" to "Based on the following graph, it is possible to say that the hydrogen bonds that exist in the water molecules are weaker in a solid state than in a liquid state".
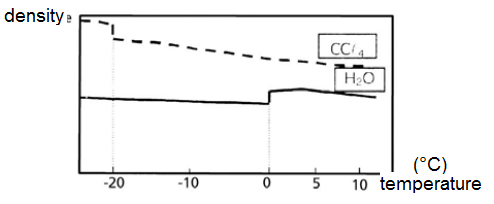
(the answer is "false")
I've read that hydrogen bonds are broken upon ebulition, for example. Can I say that there is a change in force as for hydrogen bonds in solids and liquids too?
In other words, can I say that the hydrogen bond is stronger in lliquids than in solids or vice-versa?
Could you guys please give me a hint or a link on how I can find the answer?

Thank you