Let me see if I an explain this. In the following equilibrium, the reaction goes completely to the right.
HCl + H
2O

H
3O
+ + Cl
-That means Cl
- is a weaker base than H
2O. If we repeated the reaction below, the equilibrium goes to the left.
NH
4Cl + H
2O
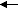
H
3O
+ + Cl
- + NH
3The explanation is the same. The equilibrium favors the weakest base (least available electrons).
If we took hydrochloric acid and added another compound to it, the equilibrium would favor formation of the weakest base. If the compound being added is more basic than water, the conjugate acid of the compound will be the major product of the equilibrium.
The question at hand does not give us the other compound specifically, but the principle is the same. If we add a compound that is a weaker base than water, but a stronger base than benzene, in which system will the greater proportion of the protonated product form? (I didn't mean to give away that the electrons of benzene are less available than water.)