The silver zinc oxide electrochemical cell, used to power hearing aids and wristwatches, is based on the following half reactions, written as a reduction:
Zn2+ + 2e- --> Zn(s) ε0 = - 0,763 V
Ag2S(s) + H2O(l) + 2e- --> 2Ag(s) + 2OH- ε0 = + 0,344 V
The table below indicates some possible relationships between the characteristics presented by this cell.
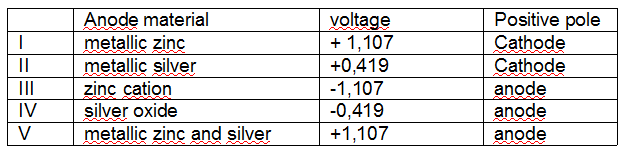
The frame line that corresponds to the correct relationship set is
(A) I.
(B) II.
(C) III.
(D) IV.
(E) V
I have no idea how to solve this. Could you guys give me a hint (or a couple of hints)? I don't get the "positive pole" part. How come it is "cathode"? Where does this come from? Isn't zinc the anode?