In a very acidic environment (pH < 1), the essential amino acid aspartic acid will look like
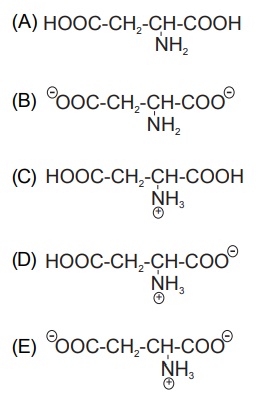
Could you guys please help me solve this one? I don't know how to start solving it. I know that "in a very acidic environment" there is a lot of H
+, but I'm stuck here.
Thank you