An aqueous solution of sucrose is put in contact with equal volume of an aqueous solution of NaCl. This results in the following equilibrium:
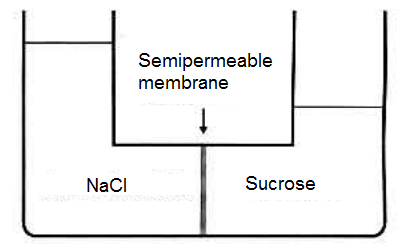
Based on the image above, one can affirm that
A) the osmotic pressure of sucrose is greater than the NaCl's osmotic pressure
B) the molality of the NaCl solution is greater than the sucrose solution;
C) the solution of NaCl has a lower boiling point than the sucrose solution
D) both solutions, when they are found at the same temperature, will have the same vapor pressure;
E) the solution of NaCl has a lower freezing point than the sucrose solution.
Could you guys guide me in this one? I thought that (B) should be true, because it seems that water is flowing towards the NaCl solution. If it is so, then the concentration (be it molarity or molality) should be higher on NaCl. But at the same time I was told the answer is (E), so I'm confused.
Or, perhaps, at the exact moment of the image, both concentrations have become equal, and so the osmotic pressure of both have become the same, meaning that all that matters for colligative properties is the Van't Hoff coefficient. Is this correct?
Thanks