several things to cover here. Let's start by taking a look at this image.
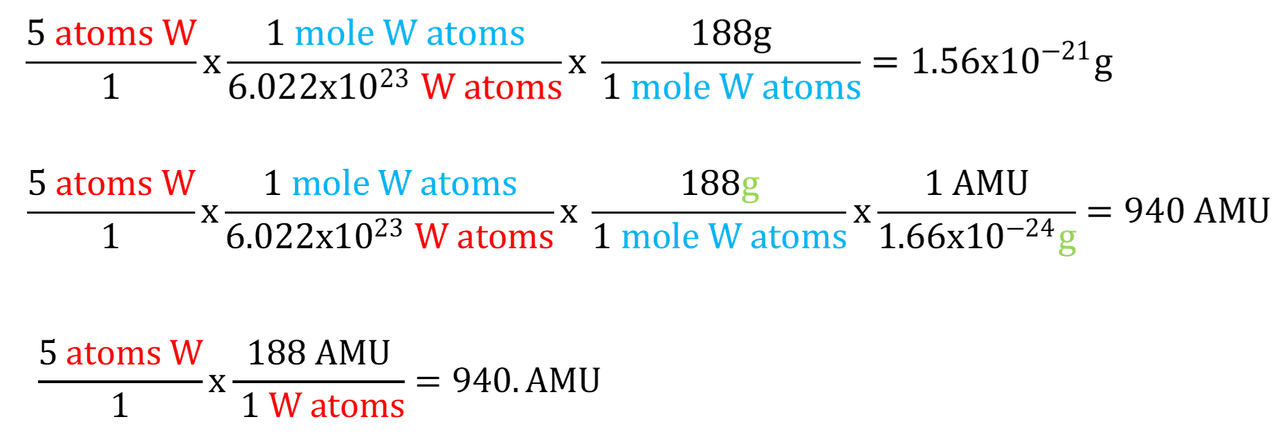
The first line is your work. The next two are mine. The color coding shows what's canceling. I think it's outstanding that you're using factor label method (aka dimensional analysis) and I encourage you to continue doing so. Your problem setup is excellent except for 1 thing. What you converted "to". The idea is you start with what you're converting from on the left and convert to the right until you have the units you are after. You want AMU, you converted to "grams" and stopped there. Why?. Check out the second line. I added another unit factor and finished the conversion to AMU's.
Now look at the third line. We already had atoms. We know the mass of 1 W-188 atom is about 188AMU so we don't need to convert to moles. Can you see the Atoms W / atoms W cancel leaving AMU's?
**********
next, let's cover sig figs. Your possible answers were
(1) 940. AMU (with 3 sig figs)
(2) 919 AMU (with 3 sig figs)
(3) 1.53x10^-21 AMU (with 3 sig figs)
(4) 187 AMU (also with 3 sig figs)
so you really only need 3 sig figs in your calculation. Actually, I encourage all my students to carry 1 extra sig fig along to avoid rounding errors. Anyway, your calcs were
5 (exact = infinite sig figs) / 6.022e23 (4 sig figs) * 188 (3 sig figs) --> 1.56x10^-21 g (3 sig figs)
now my calcs (2nd equation)
5 (exact) / 6.022e23 (4) * 188 (3) / 1.66e-24 (3) ---> 940. with 3 sig figs
now the last equation
5 (exact) * 188 (3) ---> 940. (3 sig figs)
*******
you can use more sig figs if you wish, but you really don't need more that 4 in your final answer. It's not worth looking up the isotopic mass of W-188 and doing this calc
5 (exact) * 187.958489 (9 sig figs)= 939.792445 AMU (9 sig figs)
when you're comparing it to choices with 3 sig figs... right?
you should also know that 187.958489(4) doesn't mean 187.9584894. Those masses are measured. The (4) is the standard deviation of the measurments. 187.958489(4) means 187.958489 is the average measurement and 1 standard deviation of measurements is within 4 units of the rightmost digit. i.e 67% of the measurements fell within 187.958485 to 187.958493 AMU
IF the choices were close together, then I would recommend using 1 extra sig fig and generating this answer
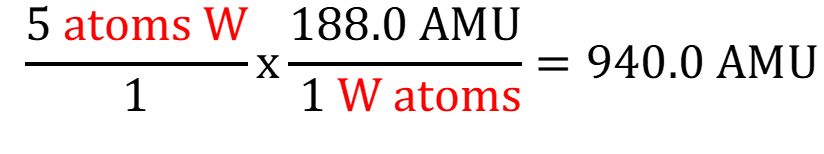
with 4 sig figs. And now we compare 940.0 to the choices. But again, not worth it in this case
************
one last thing I want to cover because I'm not sure you're crystal clear on this.
The molar mass of W-188 atoms is 187.958489 grams / mole of atoms
The atomic mass of W-188 atoms is 187.958489 AMU / 1 atom.
note the #'s are the same
note the units are different... g / 6.022x10^23 atoms... vs... AMU / 1 atom
why are the #'s the same while the units are so different? it's because of how moles of atoms (6.022x10^23 atoms) and AMU's are defined. there are 6.022x10^23 AMU's in 1 gram!. See if you can setup a conversion and from AMU / atom to g / mole of atoms.
If all else fails, remember this. you can read those masses of atoms and molecules as g / mole and as AMU / atom or AMU / molecule.
examples
copper
molar mass of Cu = 63.55 g / mole
atomic mass of Cu = 63.55 AMU / atom
molecular mass of Cu doesn't apply here.
tunsten
molar mass of W = 183.4 g / mole of W atoms
atomic mass of W = 183.4 AMU / atom
molecular mass of W doesn't apply here
isotopic mass of W-188 = 187.958489 AMU/atom
molar mass of W-188 = 187.958489 g/mol
methane
molar mass = 16.04 g/mol... 16.04g / 6.022x10^23 molecules
atomic mass doesn't apply... methane is a molecular not atomic substance
molecular mass = 16.04 AMU / molecule
make sure you're using molar mass, atomic mass, molecular mass, isotopic mass, relative atomic mass, etc correctly and that you are rock solid on the meaning of those terms!.
good luck!