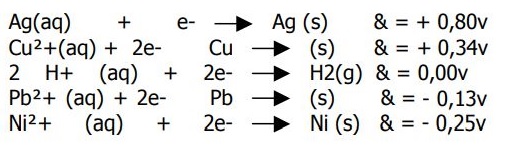
Hello. All of these reactions have their voltage on the right. I was asked "Is Ni
2+ the best reducing agent?", to which I answered "yes", and the exercise said "no."
I don't know why this is so. If nickel has the most negative reducing potential, it looks to me like it wants to oxidize. And if it wants to do so, than it is a great reducing agent.
Am I right?