I compare the heat of formation of the "reactants" (PLA) and the "products" (CO, H
2, C) to obtain the heat of reaction (here a pyrolysis or decomposition). It's a difference, not forgetting numbers of moles. Sign conventions exist, the useful part is that the present decomposition absorbs heat.
You're absolutely right that ethyl acetate has
one C too much. I botched that. "Double check" was more than rhetoric here: it's the base of science.
Methyl acetate would be the right starting material
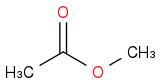
to compare with the PLA period, gratefully pinched from Wiki and appended here. Methyl acetate resembles the PLA period: same ester group, as many carbons and bonds, just two C-H becoming a C-C and an H-H, so few changes of small energy suffice.
From tables, the heat of formation of methyl acetate is ΔH=-446kJ/mol. For the liquid, not the solid, slight inaccuracy. With ethyl acetate, my error was 33kJ/mol, over the previous 214kJ/mol heat of decomposition.
The formal transformation to PLA period is the same as from two octanes becoming hexadecane and hydrogen. 2* (-250kJ/mol) becomes -456kJ/mol and 0kJ/mol, this absorbs 44kJ/mol. We could skimp on primary and secondary carbons, but I won't since this makes 5kJ/mol difference. Here we get a heat of formation ΔH=-402kJ/mol for the PLA period.
Again from tables, CO has ΔH=-110.5kJ/mol while H
2 and solid C have zero by convention. So the decomposition to 1*C + 2*CO + 2*H
2 absorbs 402-2*110.5=
181kJ/mol, not 214.
However, this is at 298K, but here the
reaction products exit at about 1500°C - I shouldn't have botched that one. Misusing at heat the capacity at room temperature (OK for H
2 and CO, not for CO
2 or H
2O)
https://en.wikipedia.org/wiki/Table_of_specific_heat_capacities8,5 + 2×29,1 + 2×28,8 = 124J/mol/K for 1*C + 2*CO + 2*H
2heating from 0 to 1500°C absorbs
further 186kJ/mol.
So from one PLA period at room temperature to 1*C + 2*CO + 2*H
2 at 1500°C, it takes about 367kJ/mol of C
3H
4O
2 weighing 72g, or
5MJ/kg.
==========
The amount of gas is easy. 2*CO + 2*H
2 at 1500°C, you can apply the ideal gas law.
The pressure is about impossible to evaluate. Your mould needs vents or it will explode, and the foam must be weak enough to open passages. The pressure results from very dynamic processes, not from an equilibrium.