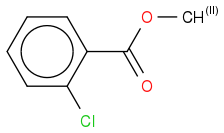
I am trying to make a fragmentation scheme from the El mass spectroscopy of methyl 2-chlorobenzoate.
 https://mona.fiehnlab.ucdavis.edu/spectra/display/JP002634
https://mona.fiehnlab.ucdavis.edu/spectra/display/JP002634 (link to the MS specter)
I belive i have found the first step where the peak goes from 170m/z to 139m/z. I belive OCH
3 is the radical in the first step.
In the next peak gap from 139m/z to 113m/z i think the carbonyl group is the radical.
And in the next peak gap from 113m/z to 75m/z i belive HCl is the next radical... Here i dont understand the reaction mechanism? Does the H come from the neighbouring carbon? And how does the ring stabilize? does it end up like this?
![C1=CC=C=C=[C+]1](https://www.chemicalforums.com/SMILES/3984b47b7db8891c4be1.png)