Interesting! Is the ketone necessary to the reaction? Two more carbons and a small spiro would inject fuss into banal compounds to serve as, well, you know.
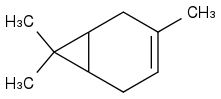
carene
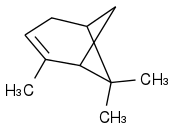
alpha-Pinene
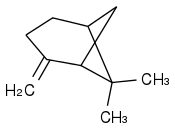
beta-Pinene
Can sodium amide be prepared in small amount at the last moment next to the main reactor? By recycling NH
3 and electrolysing molten NaBr?
Or is lithium amide advantageous?