I've spent the past day trying to figure out the structure for the given formula C
6H
12O from the given HNMR and for the life of me, I don't know if I'm doing something wrong or I just don't have a deep grasp of HNMR, but I can't figure out the structure. I think it's mainly the overlapped set that's confusing me. I read online somewhere that the 3H overlap could be a CH
2 overlapping CH or OH but I didn't learn anything like that so I'm uncertain about this, so if anyone could explain what this means, I'd really appreciate it. Thanks.
What I have so far:There should be a double bond in there somewhere since the IHD is 1.
The 3H set around 1 should be a -CH
2-
CH3The 3H set around 1.5 should be -CH3 with 1 neighboring H so either a -CH(-C)-
CH3 with no H's on the (-C) or -CH=
CH3.
The 1H set around 3.3 should be a CH with 3 H neighbors and alpha to O atom so CH
3-
CH(-C)-O- with CH's fourth substituent being a C with no H's or it could be -CH
2-
CH(-CH)-O- maybe?
The 2H set around 2ppm is either a quartet or a pentet with an overlapping 3H singlet? I'm already confused about it overlapping so I hope someone could explain what this could mean.
The closest I've gotten was
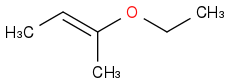
but there's a vinylic H in there so that's clearly not it...