Hydroformylation of olefins yields aldehydes which further undergo reduction to give alcohols.
[tex] H_2 + CO +RCH = CH_2 \rightarrow RCH_2CH_2CHO \tag{1}[/tex]
[tex] H_2 + RCH_2CH_2CHO \rightarrow RCH_2CH_2CH_2OH \tag{2}[/tex]
What is R in the reaction(1)?
The product doesn't seem to look alcohol
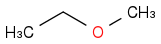
in reaction (2). What is R in reaction (2)?