ΘHello,
I am studying organic chemistry independently by working through David Klein's
Organic Chemistry as a Second Language.
In this example (attached, 3.16 in the text), is the proton on the far left or the far right of the molecule more acidic?
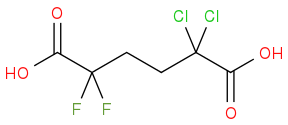
I would expect that because Cl is more electronegative than F, the C that the Cl atoms are bonded to would have a greater "partial positive" charge than the C which the F atoms are bonded to. If I'm correct, then this carbon would in turn induce a slightly partial negative charge on the C in the far-right carboxyl group, stabilizing its negative charge more than its counterpart on the left.
However the answer key tells me that I'm wrong. What am I misunderstanding?
Thank you for your suggestions!