Note: capitalization is important here, as well as correct use of indices.
This is more or less standard way of writing reaction equations.
HO
2C-C
6H
4-CO
2H is one of the isomers of the phthalic acid (there is not enough information to tell which one):
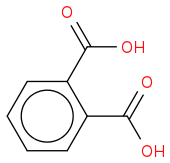
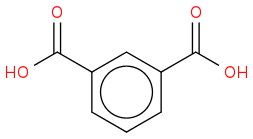
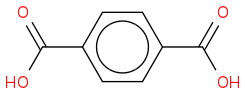
"-" doesn't actually mean anything particular, it is sometimes used to denote bonds in organic compounds, but its not really needed.
Zn(NO
3)
2 is zinc nitrate.
Notation
A + B

C
means - these on the left react producing these on the right. Typically there will be some numbers used to balance the equation (make it consistent with the real amounts of substances reacting).