Hello
I was learning about an experiment to see how much ethanol there is in a mix with gasoline.
We mix some water with salt and pour it on gasoline:
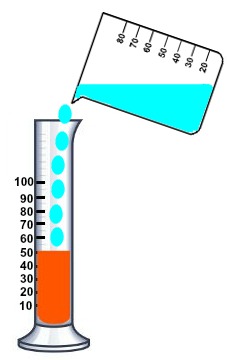
Then the little ethanol we have, since it is polar, will mix with the water with salt, and the gasoline will become pure. And then we see the volume of gasoline (the orange one) and how much it has changed to see how much ethanol we had.
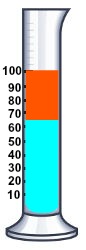
But why couldn't it be just pure water instead of water + salt? I don't understand it.