Hello all,
I have a question regarding the resonance of pyracyclene.
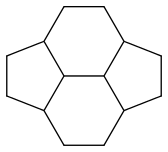
If I’m correct, pyracyclene has four resonance forms:
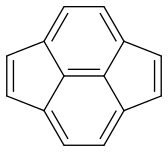
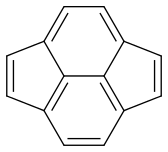
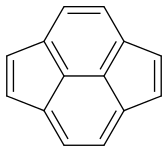
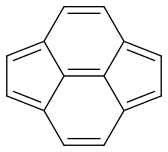
Now my problem is estimating the dominance of each form. Are they equal or is there one which is more dominant?
This is what I’ve got so far:
Rules for estimating stability of resonance structures- The greater the number of covalent bonds, the greater the stability since more atoms will have complete octets.
- The structure with the least number of formal charges is more stable.
- The structure with the least separation of formal charge is more stable.
- A structure with a negative charge on the more electronegative atom will be more stable.
- Positive charges on the least electronegative atom (most electropositive) is more stable.
- Resonance forms that are equivalent have no difference in stability and contribute equally (eg. Benzene).
Pyracyclene has the same carbon atoms, the same number of covalent bonds and a formal charge of zero. I don’t see any difference in stability either. So it seems that all forms are equivalent.
Is this correct?