It's interesting problem! I wonder whether the 3-hydroxyl indole gains as much stability as the Phenol after tautomerization? Considering that the carbonyl group and the N electron pair are already conjugated with the benzene ring and ketone is much more stable than enol. I have seen the following tautomerization

1 exist in majority in THF although 2 is aromatic.
For this
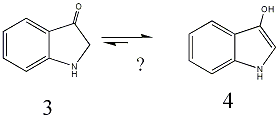
Maybe 4 exists in majority in solution, but if 3 still have the minimum concentration to react with NaBH4, then the equlibrum will move toward 3, and the reduction will be completed. Just some of my opinion