3,4-dihydroxytoluene:
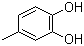
If one wanted to monoiodinate 3,4-dihydroxytoluene, the 6- position appears to be the most activated one towards EAS, since it has a -OH in para, and -Me in ortho. However, if one considers that the methyl group has some steric effect, the 4- position looks like an important product too.
Is 6-substitution the thermodynamic or kinetic product? The 4-substitution?
How does one favor the substitution in 4- instead of 6-, and how does one achieve the opposite?