If I'm not mistaken, the only isomers you can form with these compounds are stereoisomers. In these compounds, every halogen atom is bonded to the S or P atom. In the first 2 there are 6 substituents bonded to the sulphur therefore it has to be an octahedral molecule:

if you're not yet familiar with octahedral molecular geometry then familiarise yourself with it cuz octahedral molecules are easy to visualise and when you visualise them, you can easily see what kinda isomers they can have. Heres info on the isomers these kinda molecules can have:
https://secure.wikimedia.org/wikipedia/en/wiki/Octahedral_molecular_geometry#Isomerism_in_octahedral_complexesyou need at least 2 different substituents to have isomers so SF
5Cl can't have any isomers. SF
4Cl
2 can have 2 different geometric isomers. cis or trans. trans is where both chlorine atoms will be on opposite sides of the S atom like this:
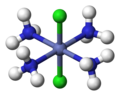
and everything else is cis:
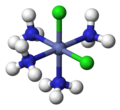
PF
4Cl has 5 substituents so it has to be trigonal bipyramidal:

As you can see trigonal bipyramidal is just octahedral with an atom removed so you treat it the same way you would an octahedral molecule. Again, you need at least 2 different substituents for there to be isomers so PF4Cl can't have any isomers. Since theres only 5 substituents of trigonal bipyramidal molecules, it can only have cis and trans though, it can't have fac and mer isomers like octahedral molecules have:

