So, my question says: Consider the following reaction at 25.0 ° C : 2NO
2 (g)
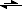
N
2O
4 (g)
The values of ΔH° and ΔS° are -58.03 kJ/mol and -176.6 J/K · mol, respectively. Calculate the value of K at 25.0° C. Assuming ΔH° and ΔS° are temperature independent, estimate the value of K at 100.0 °C.
What I thought I was supposed to do is calculate ΔG using ΔG = ΔH° - TΔS° and then plug it into the equation ΔG = -RTlnK and solve for K. But, when I did that, I kept getting -6.13 for 25°C and 6.35 for 100°C
Can you please explain to me what I am doing wrong and how to solve this problem correctly?
I know what the answers are supposed to be because my teacher provides us with the answers.
For 25°C it should be K=8.84 and for 100° C it should be 0.0798
Thank you so much in advance!