b) CaCl2 + 2HCl  CaCl42- + 2H+
CaCl42- + 2H+
Problem with balancing were not the only problem with this reaction. There is no CaCl
42- complex.
c) BaBr2 + 2HCl  BaCl2 + 2H+ + 2Br-
BaCl2 + 2H+ + 2Br-
This is wrong - but it is at least partially a problem with all your reactions. What is BaBr
2? Is it solid? Or a dissolved, undissociated molecule? And what is BaCl
2? And why there is HCl on the left, but H
++Br
- on the right? Make all these reaction net ionic, and mark what is solid, what is not.
d) BaSO4 + 2HCl  BaCl2 + H+ + HSO4-
BaCl2 + H+ + HSO4-
So now that I have these (hopefully) correct chemical equations, I need to know which of the equations will proceed the most to the right in order to find which of the four compounds will dissolve the most in HCl (as opposed to their dissolutions into water).
I am still confused on how to figure out which equation will proceed the furthest to the right.
Whenever you remove one of the dissolution products from the reaction mixture, you shift the equilibrium, don't you? For example, calcium carbonate dissolves producing two ions:
CaCO
3(s)
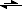
Ca
2+ + CO
32-In the presence of any acid strong enough CO
32- gets protonated:
CO
32- + H
+ 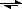
HCO
3-and the overall reaction of CaCO
3 dissolution in the presence of another acid is:
CaCO
3 + H
+ 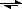
Ca
2+ + HCO
3-with the equilibrium shifted (when compared to the first CaCO
3 dissolution reaction) to the right, as protonation removes CO
32- from the solution.