Markovnikov's rule states that the nucleophile will add onto the "more substituted" carbon of the two in (what was) the double bond, and the electrophile onto the "less substituted" carbon of the two.
Firstly, does this mean alkyl substituents only, or any atom besides H? I somehow doubt it would be the latter.
Secondly, if both C atoms are substituted with the same number of substituents (of each type, if the starting atom of the substituent makes a difference), do we expect a mixture of products, regardless of whether one alkyl substituent is heavier than the other or not?
Example:
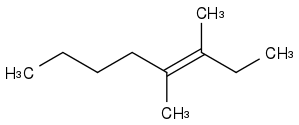
- is there a single product we can predict, or will it be a mixture?
Yes I know this is a matter of carbocation stability and to restate this question in the terms of carbocation stability is trivial. I can do so if you really wish. But I can't find this material anywhere through searching (Wikipedia does not appear to have it).