That's a circular post. Either that or you need to frame your question more clearly.
Let's take a real example rather than speaking in abstractions.
The solubility product of silver chloride in 25 C water is 1.77 × 10
−10.
This is for the process AgCl(s)
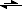
Ag
+(aq) + Cl
-(aq)
What this practically means is that at saturation the concentrations of both the Ag
+ and Cl
- ions will be 1.33 x 10
-5 mol/L. The stoichiometric equivalent of solid AgCl that would give rise to this concentration of dissociated ions in 1 L of water is about 1.9 mg. If you add less than 1.9 mg of solid AgCl to 1 L of water, it will all dissolve in stoichoimetric ratio, but none of the ionic concentrations will obviously reach the "maximum" level specified by the solubility product equilibrium constant. If you add more than 1.9 mg of solid AgCl to 1 L, the solution will saturate, meaning 1.33 x 10
-5 mol/L of Ag
+ and Cl
- ions will form in solution from 1.9 mg of the AgCl. Any solid AgCl above the 1.9 mg will remain as a solid and settle to the bottom of the flask. If you add more solid AgCl later, it, too, will settle to the bottom of the flask.
However, this still is an equilibrium constant, so the usual rules apply. If you add sodium chloride to a saturated solution, this will shift the equilibrium to the left, because now there are more chloride ions available to "react" with the available silver ions. If you raise the temperature, the equilibrium will shift to the right. If you change the particle size of the solid to very small (talking nano-regime), this will also impact the equilibrium, although the reason, and the effect, is complicated.
And like all equilibrium situations, the value of the solubility product under any condition is related to thermodynamics - the respective enthalpic and entropic changes (combined as the Gibbs energy change) that occur during dissolution. Unfortunately predicting solubility of ionic salts from thermodynamic considerations is not always easy due to the number of factors involved, so solubility remains a largely empirical phenomenon.
Here is some more about solubility products:
http://www.chemguide.co.uk/physical/kspmenu.html#top