I'm having a basic org chem amnesia moment: If I were to condense
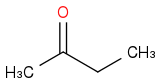
and
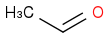
acid catalyzed, would I expect to see (Product a)
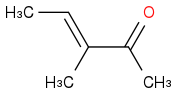
or (Product b)
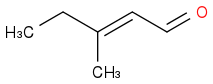
or both.
If both, any way to estimate (roughly) the ratios or which would be the dominant product?
I think I ought to get (Product a) but not sure if the other crossed Product (b) will form too.
I'm assuming I did get the crossed products right. I hope.