Have to tried anything on this?
I have had some interesting times with indole, it can behave like an aromatic compound in some reactions but often the N-H creates issues as this is slightly acidic (pKa 21).
You may be able form the dimethylborate of indole by first reacting indole with Et-MgBr to form
![Br[Mg]n2ccc1ccccc12](https://www.chemicalforums.com/SMILES/22bb0830bb4197b3a387.png)
When this acts as a nucleophile it does so at the 3-position (at least in alkylation and acylation reactions)
You should be able to react this with trimethylborate to form the dimethylboronic ester. Acid hydrolysis should yeild the desired boronic acid.
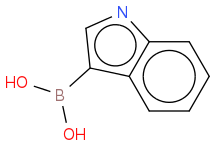
I don't know if this will work but this is the best I can offer. (For some reason the SMILES has aromatised the indole and omitted the proton from the nitrogen but I hope you get the general idea)