Lets say I wanted to make something like this:
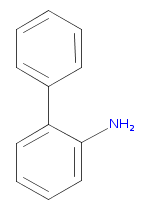
I know there are many ways to make this, but I'm wondering if I could make it via a Kolbe electrolysis. I've only heard of Kolbe electrolysis being used for dimerisations, but would it be possible to control the stoichiometry and addition of your reagents in such a way to produce an adduct like this?
If I just added the reagents normally, I'm guessing I'd get a mixture of 3 different products which would drop my yield and be a pain in the ass to seperate, so I'm looking for a way to make the reaction favour my product. And BTW, that compound is just an example, its not what I want to synthesise, I'm just looking into this class of reaction.