Hi guys,
maybe bit stupid question but:
"How many peaks are in NMR spectra of butanol"
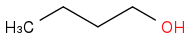
I think its 4, 1 for CH
3 group, 1 for OH, 1 for the CH
2 to which OH bonds and 1 peak for the 2 CH
2 groups in the middle.
If we dont count isomers or they didnt mean C NMR.
Am I right?
Thanks