Hi,
I'm studying chemistry, but this year I had a Chemical Engineering subject, and today was the final exam, and in it, there was an mass balance exercise which I believe it's imposible to solve without more data.
This is the diagram:
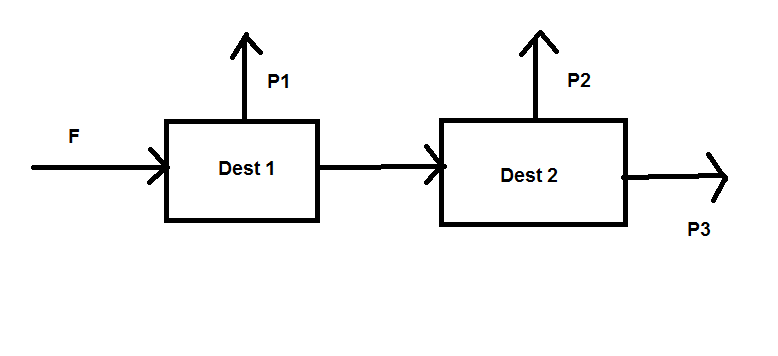
1 initial flowing, and just 2 distillators...
The data they gave is the following:
Compsition of F (100kg/h):
20% of A
30% of B
50% of C
About P1:
It has the 75% of F's A total mass (i.e. 0.75*20= 15 kg)
B concentration in P1: 5%
About P2:
It has the 80% of F's B total mass (i.e. 0.8*30= 24 kg)
C concentration in P1: 4%
About P1:
It has the 80% of F's C total mass (i.e. 0.8*50= 40 kg)
A concentration in P1: 1%
And thats it.
Not even the total mass of P1, P2 or P3 or in the intermediate flowing.
The exam had 3 exercises, I solved correctly 2 of them in 30 minutes, and I spent 2:30h trying to figure how this one was, but I can't see a way.
I got a bunch of balance equations, but none of them gave me results, I think you need more data to solve this.
I'll talk to the professor tomorrow morning, but I'd really hope you could help me out with this.
Regards