I plugged the correct values 1.82 and 0.75 Å into equation 2° and I got a volume of 27Å3 which is bigger than the one calculated using the edge length of the octahedron. Seems like the error where I overview the packing efficiency isn't the only one. Something else was wrong there, too and I don't know what  .
.
Of course. Your octahedron doesn't really contain one full atom of both. That's why. Look at this figure.
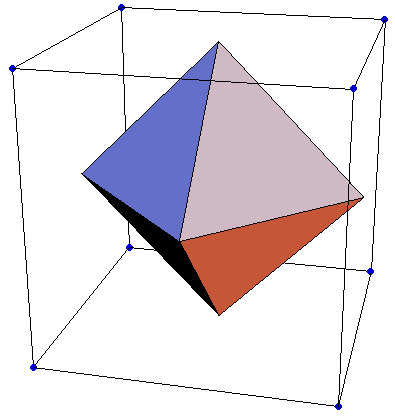
Why would you think one full atom of Li & Cl is fully inside the octahedron?
If you did want to do this try for the full cube. It ought to have one full Li atom & 8 vertex Cl- atoms (each contributing 1/8th ) plus 6 face center Cl- atoms (each contributing 1/2).
That's a total of 1 Li & 1+3 = 4 Cl atoms. The volume together is 1(1.76) + 4(25.2)≈102 Å
3Compare to cube volume = 5.14
3 =136 Å
3That'd yield a void fraction of 34/136 = 0.25. Pretty close to what's expected I'd hope?