Hi,
Could someone please provide me some insight as to how to solve the following problems?
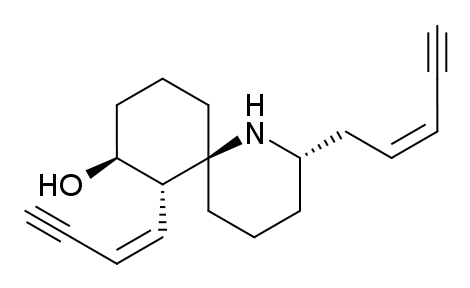
1. If histrionicotoxin A (shown above) were treated with pyridinium chlorochromate (PCC) the resulting product would contain the following new functional group:
a. diol
b. alcohol
c. carbonyl
d. alkene
e. amide
Shouldn't the alcohol turn into a ketone? How come ketone is not one of the answers listed?
2. Please see attachment below. I'm not sure where to start and have tried researching this topic on my own already.

Thank you in advance!