Hello there!
I'm working to produce a cost-benefit analysis of some chemical treatments. I'm having trouble with my math, and I'd really appreciate some help. Thanks in advance!
Short version:I'm using an Excel spreadsheet to run equilibrium calculations, and I'm having trouble with acid dissociations.
I'm starting with 12 mg/L of total Phosphorus contained in the water, the vast majority of which is PO
4. Because the pH is stable, I'm trying to find out how much of each ion is present.
(Phosphorus is multiprotic and can dissociate into H
2PO
4(-) HPO
4(-2) PO
4(-3) so there's a nice big mess here.)
I'm trying to put together formulas in Excel that can find the concentrations of each ion, but for the life of me I can't figure it out. Here's what I have so far:
(H(+)+Δ)(Δ)/(HPO4-Δ)=pKa
Here, Δ represents the reaction unit that dissociates.
Problem is, I'm not really able to solve for Δ here. Because pH is stable, I'm treating H
(+) like a constant. My gut tells me this should be quickly solvable and easily modeled with a little bit of Excel, but I'm just not seeing it.
Long version:This is the reaction for the formation of
struvite, a nasty hard salt we don't want clogging up our pipes.
Mg(+2) + NH4(+) + PO4(-3)  MgNH4PO4
MgNH4PO4 Current plan is to starve this reaction of phosphate by adding ferric sulfate, which should swap ions for ferric phosphate.
Fe(+3) + PO4(-3) 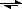 FePO4(s)
FePO4(s) This has a few other added bonuses, like bringing the pH down (struvite becomes more soluble in lower pH) and preventing other nasties like H
2S from forming.
Overall, these reactions are occurring at 4°C in biologically active water that's saturated in CO
2 and varies from 8-7 in pH. I can give more details if they're needed. (The CO
2 part is particularly nasty, because it means any time the CO
2 is gassed off, the pH rises and struvite forms. CO
2 is gassed in turbulent areas, like the inside of our big expensive pumping stations.)
My goal is to find an ideal dosage amount that could inhibit struvite formation and help dissolve existing struvite in the long term. From there, I can provide cost-per-million-gallons and annual cost estimates.