I was looking for some help with an electrochem question, shown below:
"A current of 0.25 A is passed through 400 mL of a 2.00 M solution of NaCl for 35.0 minutes. What will be the pH of the solution after the current is turned off?"
First off, I want to know how many moles are in this solution so I can use that as a conversion factor later on if necessary.
2.0 mol / 1000 mL = .40 mol / 200 mL = .80 mol / 400 mL
I figure a good next step is writing the chemical equation.
NaCl
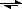
Na
+ + Cl
-I also know that in my chemistry class (AP chemistry), we can assume complete dissociation. Therefore
both Na
+ and Cl
- have a concentration of 2.0 M at equilibrium.
Because there is an acid/base component of the question, I know I need a reaction involving molarity of H
+ so I can eventually get pH. I can do this by writing a chemical reaction involving acid.
Cl
- + H
3O
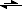
HCl + H
2O
Now here's where I start to get lost. I know that I should be using the information about the electrical current, but I'm not sure how to do it. I've made two attempts so far:
Attempt 1: (.80 mol / .400 L) * (96845 C / mol) * (.25 C / sec) * (1 min / 60 sec) * (1 /35 min)
The problem here is that the units on the answer are L
-1, while I'm looking for mol / L.
Attempt 2: (.25 C / sec) * (60 sec / min) * (35 min / 1)
The problem here is that I end with mol, not mol / L AND this doesn't incorporate the information I have about the original concentration.
I've also considered doing an ICE problem, but I wouldn't know how to incorporate the electrochemical data.
Could anyone help point me in the right direction? Thanks!