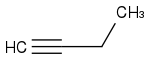
Hello, I had a quick question on this molecule regarding the number of carbon-carbon s bonds ?
Does s bond refer to the s orbital direct overlap bond, so a sigma bond ? and If so does that mean there are 3 (s or sigma) bonds because 1 from the triple bond, 1 from the second carbon to the third carbon, and 1 from the 3rd carbon to the 4th carbon for a total of 3 sigma bonds ? any help is appreciated just started an orgo class over the summer.