I understand that formal charge is a charge associated with an atom when it doesn't exhibit the expected # of valence e-.
However, how can you assume that the middle carbon isn't donating 2 of its valence electrons to the bond here rather than the oxygen having an extra electron?
The picture is incorrect, struggling with formatting. It should be just 'O' with a formal charge -1, not HO.
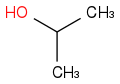
Very appreciative for feedback...