Hi, the question goes like that:
How would you separate a mixture of benzoic acid, p-nitroaniline, azobenzene, and ethylbenzene, using acid base extraction.
My attempt: well, since all the compounds are aromatic ones, i would begin by dissolving the mixture in some non-polar organic solvent, like benzene (it should be non-polar so that the salts will go to the aqueous phase). Then, add some NaOH, which will react with the benzoic acid to give sodium benzoate that will go to the aqueous phase. Separate the aqueous phase. Then add some HCl, which will protonate the nitroaniline, and it will be transferred to the aqueous phase. Again, separate the aqueous phase. And here i got stuck. I am left with azobenzene and ethylbenzene. As far as i think, the first is basic because of the non-bonding electrons on the nitrogens. So if it is basic, it would be protonated just like nitroaniline when acid is added, and i have no idea how to separate those two. On the other hand, if it's neither acidic nor basic, how can i separate it from ethylbenzene? Could it have something to do with H
2SO
4?
Azobenzene:

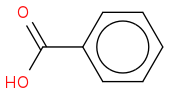
benzoic acid:
p-nitroaniline:

ethylbenzene:

Thanks